特惠-26考研冲刺
特惠-27考研课
双证-在职硕士
免联考-同等学力
复试分数线
26复试全面指导
模拟复试面试
26考研-全套真题
26考研估分
保研-路线图
27考研-智能择校
27考研-英语测评
27考研-新大纲对比
热门-计算机择校

扫码加入训练营
牢记核心词
学习得礼盒
2018考研复习进行时,考研英语阅读真题中的文章,多摘自英美主流外刊,有时候你认识所有单词、搞清全部语法还不够,还需要了解英美文化,掌握他们的表达方式,这就是阅读的潜台词。下面新东方在线考研整理《2018考研英语双语阅读精选》,速来学习吧!
Experimental HIV vaccine regimen is well-tolerated, elicits immune responses
艾滋病疫苗真来了?强生宣布:志愿者100%产生抗体
Results from an early-stage clinical trial called APPROACH show that an investigational HIV vaccine regimen was well-tolerated and generated immune responses against HIV in healthy adults. The APPROACH findings, as well as results expected in late 2017 from another early-stage clinical trial called TRAVERSE, will form the basis of the decision whether to move forward with a larger trial in southern Africa to evaluate vaccine safety and efficacy among women at risk of acquiring HIV.
一项称作“APPROACH”前期临床试验结果表明,一种试验性的艾滋病毒疫苗疗法在健康的成年人中耐受性良好,并能够对HIV产生免疫反应。APPROACH方法以及2017年年底另一项名为“TRAVERSE”的临床试验结果,将决定是否在南部非洲进行更大规模的试验,以评估疫苗的安全性和其对有感染艾滋病风险的妇女的疗效。
The APPROACH results will be presented July 24 at the 9th International AIDS Society Conference on HIV Science in Paris.
该方法的研究成果将于7月24日在巴黎举行的第九届国际艾滋病社会会议上公布。
The experimental vaccine regimens evaluated in APPROACH are based on "mosaic" vaccines designed to induce immunological responses against a wide variety of HIV subtypes responsible for HIV infections globally. Different HIV subtypes, or clades, predominate in various geographic regions around the world. The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, funded pre-clinical development of these vaccines. Together with other partners, NIAID supported the APPROACH trial, which is sponsored by Janssen Vaccines & Prevention B.V., part of the Janssen Pharmaceutical Companies of Johnson & Johnson. The manufacture and clinical development of the mosaic vaccines are led by Janssen.
“APPROACH”评估的实验性疫苗方案是基于“马赛克”疫苗,该疫苗旨在针对全世界引起HIV-1感染的不同HIV病毒亚型产生免疫原。不同的艾滋病毒亚型以及病毒分支,在世界各地的不同地理区域占主导地位。国立变态反应与传染病研究所是国家卫生研究院的一部分,资助了这些疫苗的临床前开发。该研究所与其他合作伙伴对该方法的试验提供了一定的支持,该试验由詹森疫苗与预防中心赞助,该中心是强生公司詹森制药公司的一个部门。这些马赛克疫苗的制造和临床开发由詹森公司发起。
A safe and effective HIV vaccine would be a powerful tool to reduce new HIV infections worldwide and help bring about a durable end to the HIV/AIDS pandemic, said NIAID Director Anthony S. Fauci, M.D. "By exploring multiple promising avenues of vaccine development research, we expand our opportunities to achieve these goals."
“安全有效的HIV疫苗将成为减少全球范围内艾滋病毒感染新增的强有力的工具,并有助于永久终结艾滋病毒/艾滋病的流行感染。” 国立变态反应与传染病研究所的主管安东尼.福奇博士说“通过探索多种具有开发前景疫苗的研究方法,我们扩大了实现这些目标的机会。”
APPROACH involved nearly 400 volunteers in the United States, Rwanda, Uganda, South Africa and Thailand who were randomly assigned to receive one of seven experimental vaccine regimens or a placebo. APPROACH found that different mosaic vaccine regimens were well-tolerated and capable of generating anti-HIV immune responses in healthy, HIV-negative adults. Notably, the vaccine regimen that was most protective in pre-clinical studies in animals elicited among the greatest immune responses in the study participants. However, further research will be needed because the ability to elicit anti-HIV immune responses does not necessarily indicate that a candidate vaccine regimen can prevent HIV acquisition.
来自美国、卢旺达、乌干达、南非和泰国的近400名志愿者被随机接种7个实验性疫苗方案或安慰剂中的异种。实验研究方法发现不同的疫苗方案耐受性良好,并能在健康的、HIV呈阴性的成年人中产生抗艾滋病毒的免疫反应。值得注意的是,在前期动物临床研究中发现的最具保护作用的疫苗方案在临床人体实验中也获得了最大的免疫反应。然而,试验还需要进一步的研究,因为获得抗艾滋病毒免疫反应的能力并不一定表明候选疫苗方案就可以预防艾滋病毒。
According to the researchers, the findings from APPROACH, as well as from animal studies, support further evaluation of a lead candidate regimen in a clinical trial to assess its safety and efficacy. Plans for such a clinical trial to be conducted in southern Africa are in development, with projected enrollment of 2,600 healthy, HIV-negative women. Should the larger trial move forward, it is expected to begin enrollment in late 2017 or early 2018.
根据研究人员的说法,“APPROACH”的实验结果以及在动物身上研究的试验结论对进一步评估主要候选方案的安全性和有效性能够提供一定的支持。在南部非洲进行这种临床试验正在计划中,预计将有2600名健康的HIV阴性妇女参加。更大规模的尝试推进试验,预计将于2017年末或2018年初提上日程。
In APPROACH, study participants received four vaccinations over 48 weeks: two doses of an initial, or "prime," vaccine, followed by two doses of a booster vaccine. The experimental regimens all incorporated the same vaccine components in the prime vaccination, known as Ad26.Mos.HIV. The vaccine uses a strain of common-cold virus (adenovirus serotype 26, or Ad26), engineered so that it does not cause illness, as a vector to deliver three mosaic antigens created from genes from many HIV variants. The booster vaccination included various combinations of the Ad26.Mos.HIV components or a different mosaic component, called MVA-Mosaic, and/or two different doses of clade C HIV gp140 envelope protein containing an aluminum adjuvant to boost immune responses.
在研究中,志愿者在48周内接受了四次接种疫苗:两剂初始的,或“优质”的疫苗,其次是两剂增强疫苗。这些实验方案都在疫苗接种中加入了相同的疫苗成分,即Ad26. Mos艾滋病毒。该疫苗使用的是一种不致病的普通感冒病毒(腺病毒血清型26),该病毒可作为多种多HIV变种基因中产生的三种嵌合体抗原的载体。加强疫苗接种包括各种组合的Ad26. Mos 艾滋病毒成分或不同的马赛克元素,称为“马赛克”嵌合型疫苗,或两种不同剂量的含有一种铝佐剂来增强免疫反应的衍生C HIV gp140信封蛋白。
The Ad26-based mosaic vaccines were initially developed by the laboratory of NIAID grantee Dan H. Barouch, M.D., Ph.D., and Janssen. In pre-clinical studies, regimens incorporating these mosaic vaccines protected monkeys against infection with an HIV-like virus called simian human immunodeficiency virus (SHIV). The most effective prime-boost regimen reduced the risk of infection per exposure to SHIV by 94 percent and resulted in 66 percent complete protection after six exposures. Researchers identified and characterized the vaccine-induced immune responses that correlated with this protection.
基于Ad26的马赛克疫苗最初是由国立变态反应与传染病研究所授权的Dan h . Barouch博士和詹森公司开发的。在前期临床研究中,纳入这些马赛克疫苗的方案保护了猴子免受感染,这种病毒被称为类人猿免疫缺陷病毒。最有效的原动力疗法降低了感染的风险,降低比例达到了94%,且在6次至于病毒感染下得到了66%的完全保护。研究人员发现并描述了与这种保护相关的疫苗诱导的免疫反应。
The promising, early-stage results from the APPROACH study support further evaluation of these candidate vaccines to assess their ability to protect those at risk of acquiring HIV, said Dr. Barouch, a principal investigator for APPROACH. He also is director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center in Boston and professor of medicine at Harvard Medical School.
“这种具有开发前景的APPROACH研究成果能够为进一步评估候选疫苗对有感染艾滋病风险人的保护能力提供支持,”Barouch博士说,他是APPROACH研究的首席研究员。他也是位于波士顿以色列女执事医疗中心的病毒学和疫苗研究中心的主任,也是哈佛医学院的医学教授。
Following the third vaccination, most APPROACH participants had developed antibody and cellular immune responses against HIV. The different boost vaccines altered the magnitude and character of these immune responses, with the regimen that showed greatest protection in monkey studies also eliciting among the greatest immune responses in humans. The anti-HIV immune responses increased after the fourth vaccination.
在第三次疫苗接种之后,大多数参与者都已经产生抗体,并通过细胞免疫反应来对抗HIV病毒。不同的接种疫苗对免疫反应的大小和特征也有一定的影响。那些在猴子身上产生最大免疫反应的疫苗在人类身上也出现了相同的效果。第四个疫苗接种后,抗艾滋病毒免疫反应增加。
The researchers conclude that further evaluation of this approach would use a regimen comprising two Ad26 mosaic primes and two boosts with Ad26 mosaic and clade C gp140. The ongoing TRAVERSE trial is comparing Ad26-based regimens containing three mosaic antigens (trivalent) with Ad26-based regimens containing four mosaic antigens (tetravalent). Results from TRAVERSE are expected in late 2017.
研究人员得出结论,对这种方法的进一步评估将采用两种Ad26马赛克优质型和两种Ad26马赛克增强型以及clade C gp140型。目前正在进行的TRAVERSE试验正在比较含三种Ad26基的马赛克抗原以及含4中Ad26基的马赛克抗原的效果。试验结果预计将在2017年年底公布。
来源:爱语吧
【英语阅读资料】这里有↑↑↑

 资料下载
资料下载
2014年-2025年考研历年真题汇总
发布时间:2024-04-25扫码添加【考研班主任】
即可领取资料包
考研大纲PDF电子版下载-历年(附解析)
发布时间:2024-04-25扫码添加【考研班主任】
即可领取资料包
2026年考研政数英备考资料zip压缩包
发布时间:2024-04-25扫码添加【考研班主任】
即可领取资料包
考研英语大纲词汇5500打印版(基础必备)
发布时间:2024-04-25扫码添加【考研班主任】
即可领取资料包
新东方在线考试模拟题【12套】
发布时间:2024-04-25扫码添加【考研班主任】
即可领取资料包
2026年考研专业课知识点总结
发布时间:2024-04-25扫码添加【考研班主任】
即可领取资料包
新东方考研资料下载地址
发布时间:2023-05-17新东方在线考研资料合集
下载方式:微信扫码,获取网盘链接

目录:
1.2013-2023年近10年政数英真题及解析PDF版(新东方)
2.2013-2023年专业课考试历年真题及解析PDF版
3.24考研复习备考资料大合集:大纲+备考资料+词汇书+考前押题+自命题
资料介绍:
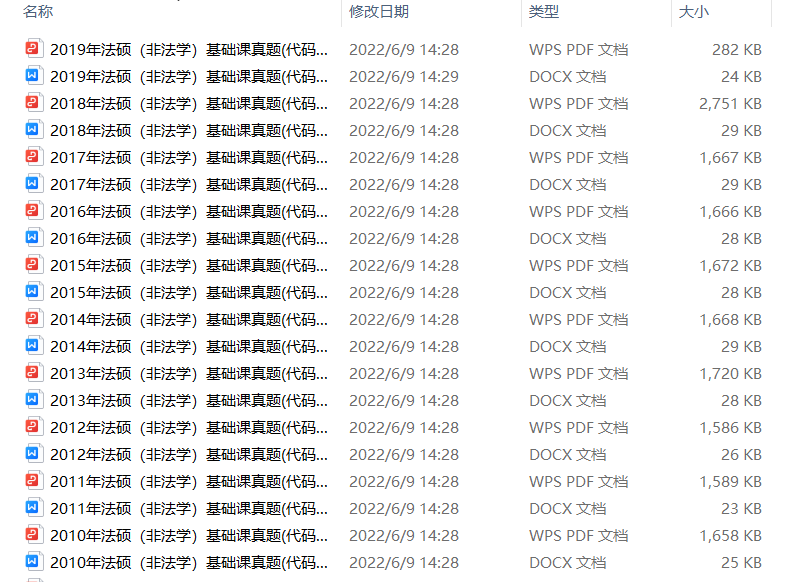
1.2013-2023年近10年政数英真题及解析PDF版(新东方)
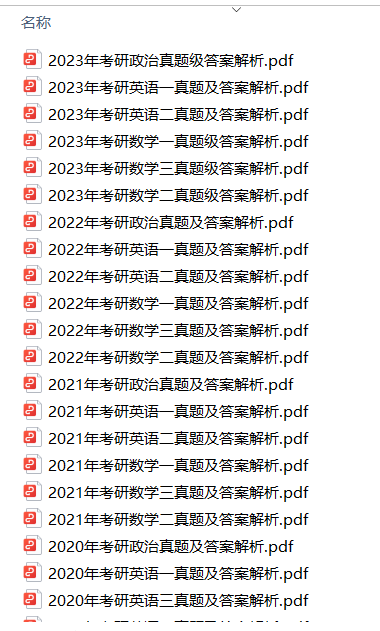 、
、
2.2013-2023年专业课考试历年真题及解析PDF版
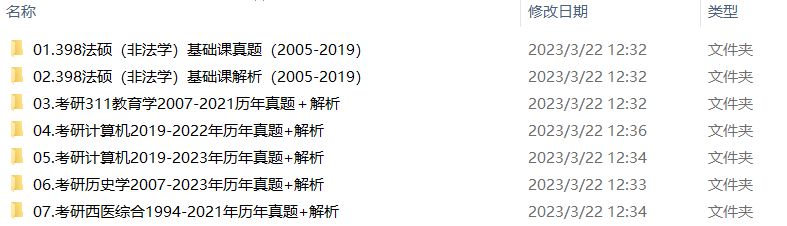
3.24考研复习备考资料大合集
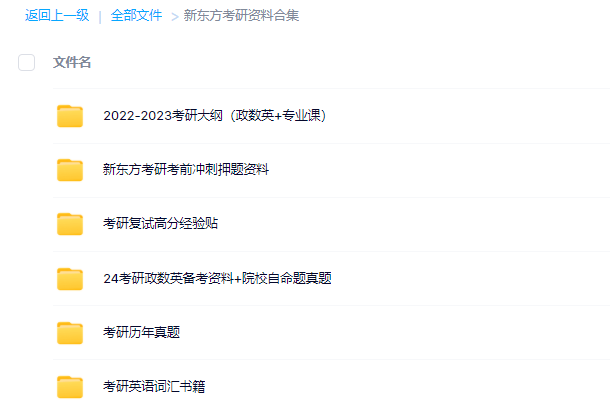
3.24考研复习备考资料:考研大纲
3.24考研复习备考资料:政数英备考资料+自命题真题
------------------
考研备考过程中,尤其是专业课部分,参考往年的考试真题,对于我们的复习有更好的帮助。北京大学考研真题资料都有哪些?小编为大家进行了汇总。
北京大学考研真题资料-公共课
北京大学考研真题资料-专业课


以上就是关于“北京大学考研真题资料下载(历年汇总)”的整理,更多考研资料下载,请关注微信获取下载地址。
2024考研公共课必背知识点汇总
发布时间:2023-01-03扫码添加【考研班主任】
即可领取资料包
2013-2023考研历年真题汇总
发布时间:2023-01-03扫码添加【考研班主任】
即可领取资料包
考研英语大纲词汇(PDF可打印)
发布时间:2023-01-03扫码添加【考研班主任】
即可领取资料包
2024考研专业课知识点总结
发布时间:2023-01-03扫码添加【考研班主任】
即可领取资料包
2023考研政治 内部押题 PDF
发布时间:2022-11-16扫码添加【考研班主任】
即可领取资料包
徐涛:23考研预测六套卷
发布时间:2022-11-16扫码添加【考研班主任】
即可领取资料包
考研政数英冲刺资料最新整理
发布时间:2022-11-16扫码添加【考研班主任】
即可领取资料包
23考研答题卡模板打印版
发布时间:2022-11-16扫码添加【考研班主任】
即可领取资料包
2023考研大纲词汇5500PDF电子版
发布时间:2022-07-28扫码添加【考研班主任】
即可领取资料包
考研历年真题(公共课+专业课)
发布时间:2022-07-28扫码添加【考研班主任】
即可领取资料包
考研英语阅读100篇附解析及答案
发布时间:2022-01-07扫码添加【考研班主任】
即可领取资料包
新东方考研学霸笔记整理(打印版)
发布时间:2022-01-07扫码添加【考研班主任】
即可领取资料包
2001-2021年考研英语真题答案(可打印版)
发布时间:2022-01-07扫码添加【考研班主任】
即可领取资料包
考研英语词汇5500(完整版下载)
发布时间:2022-01-07扫码添加【考研班主任】
即可领取资料包
2022考研政审表模板精选10套
发布时间:2022-01-07扫码添加【考研班主任】
即可领取资料包
历年考研真题及答案 下载
发布时间:2021-12-09扫码添加【考研班主任】
即可领取资料包
考研政审表模板汇总
发布时间:2020-06-17扫码添加【考研班主任】
即可领取资料包
近5年考研英语真题汇总
发布时间:2020-06-17扫码添加【考研班主任】
即可领取资料包
考研英语大纲词汇5500
发布时间:2020-06-17扫码添加【考研班主任】
即可领取资料包
2022考研12大学科专业排名汇总
发布时间:2019-11-21扫码添加【考研班主任】
即可领取资料包
2023考研政治复习备考资料【珍藏版】
发布时间:2019-11-21扫码添加【考研班主任】
即可领取资料包
考研英语万能模板+必备词汇+范文
发布时间:2019-11-21扫码添加【考研班主任】
即可领取资料包
考研数学一、二、三历年真题整理
发布时间:2019-11-21扫码添加【考研班主任】
即可领取资料包

添加班主任领资料
添加考研班主任
免费领取考研历年真题等复习干货资料

 推荐阅读
推荐阅读
为了让考研的同学更高效地复习考研英语,新东方在线考研频道整理了“考研英语1阅读错几个后的复习计划”,考研的同学可以了解一下,希望对大家有所帮助。
为了让考研的同学更高效地复习考研英语,新东方在线考研频道整理了“考研英语二阅读篇数及题型分析”,考研的同学可以了解一下,希望对大家有所帮助。
为了让考研的同学更高效地复习考研英语,新东方在线考研频道整理了“考研英语阅读理解的总结与反思”,考研的同学可以了解一下,希望对大家有所帮助。
来源 : 网络 2025-06-13 08:02:00 关键字 : 考研英语阅读理解
为了让考研的同学更高效地复习考研英语,新东方在线考研频道整理了“提高考研英语一阅读理解的五大策略”,考研的同学可以了解一下,希望对大家有所帮助。
为了让考研的同学更高效地复习考研英语,新东方在线考研频道整理了“探索考研英语阅读文章的逻辑结构”,考研的同学可以了解一下,希望对大家有所帮助。
来源 : 网络 2025-06-12 08:03:00 关键字 : 考研英语阅读

 资料下载
资料下载
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
新东方在线考研资料合集
下载方式:微信扫码,获取网盘链接

目录:
1.2013-2023年近10年政数英真题及解析PDF版(新东方)
2.2013-2023年专业课考试历年真题及解析PDF版
3.24考研复习备考资料大合集:大纲+备考资料+词汇书+考前押题+自命题
资料介绍:
1.2013-2023年近10年政数英真题及解析PDF版(新东方)
 、
、
2.2013-2023年专业课考试历年真题及解析PDF版


3.24考研复习备考资料大合集

3.24考研复习备考资料:考研大纲

3.24考研复习备考资料:政数英备考资料+自命题真题

------------------
考研备考过程中,尤其是专业课部分,参考往年的考试真题,对于我们的复习有更好的帮助。北京大学考研真题资料都有哪些?小编为大家进行了汇总。
北京大学考研真题资料-公共课

北京大学考研真题资料-专业课


以上就是关于“北京大学考研真题资料下载(历年汇总)”的整理,更多考研资料下载,请关注微信获取下载地址。
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包
扫码添加【考研班主任】
即可领取资料包

 阅读排行榜
阅读排行榜
 相关内容
相关内容